If an atom has an atomic number of 15 and an atomic mass of 31, how many protons, neutrons and electrons does the atom contain?

Each element is identified by the number of protons in its atoms. This number is the atomic number. The periodic table lists the elements in order of increasing atomic number. Each element has a symbol, which is one or two letters. The first letter is always capitalized. If there is a second letter, it is lowercase. 15: Phosphorus: P: For chemistry students and teachers: The tabular chart on the right is arranged by Atomic number. The first chemical element is Hydrogen and the last is Ununoctium. Please note that the elements do not show their natural relation towards each other as in the Periodic system. The number after the 'N', 15, is the mass number. The mass number is the sum of the number of protons and the number of neutrons. So, if you find a periodic table, you can find the atomic number.
1 Answer

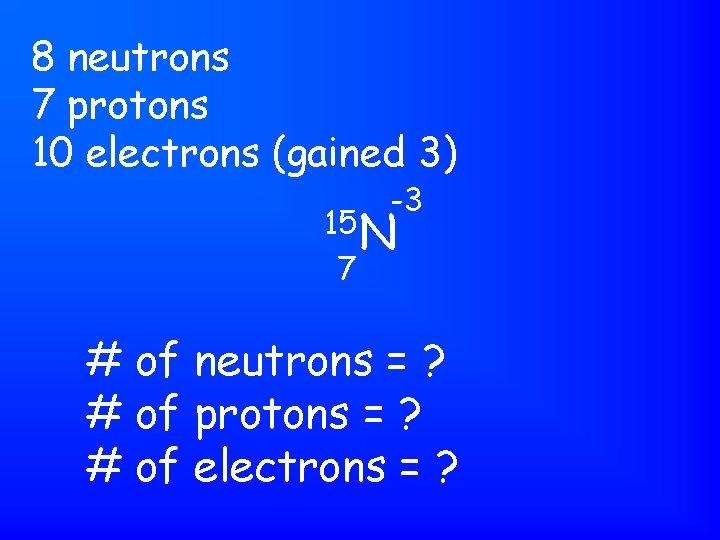
This is the isotope phosphorus-31. It has 15 protons, 15 electrons, and 16 neutrons.
Atomic Number 15 Belongs To Which Group
Explanation:
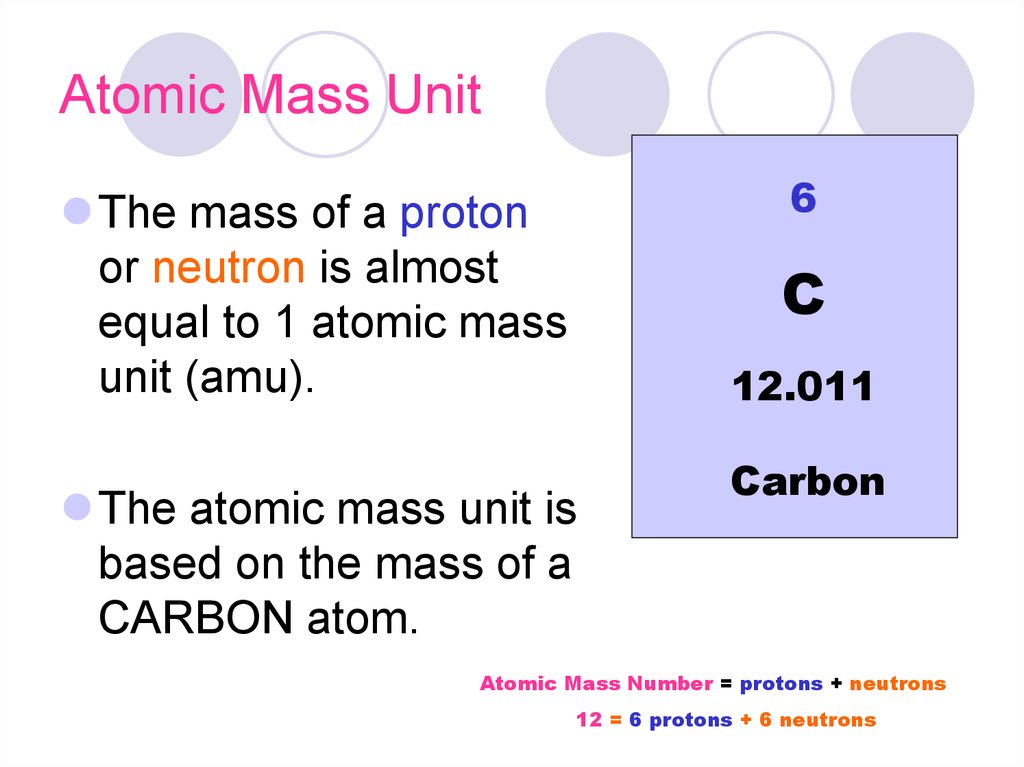
The atomic number for any element is the number of protons in its atomic nuclei. In a neutral atom, the number of electrons are the same as the number of protons. The atomic mass (mass number) is the sum of the protons and neutrons in the nucleus. Mass number can vary due to the fact that the number of neutrons can vary. Isotopes are named for their mass numbers.
So, this element has 15 protons (atomic number), 15 electrons, and 16 neutrons (mass number minus atomic number).
This isotope is phosphorus-31, which has atomic number 15.
Atomic Number 15 Mass Number 32
Related questions
